Minnesota Asbestos Exposure Lawyers
Asbestos exposure in Minnesota is prevalent in the mining and manufacturing industries. It has caused the deaths of thousands of Minnesotans from asbestosis, mesothelioma, lung cancer, and other cancers. Asbestos exposure may occur as unintentional contamination of mined products like taconite, asbestos in Minnesota’s power plants, or through the deliberate use of asbestos in manufacturing. In either case, the companies that mined and produced asbestos-tainted materials are liable. Our trusted mesothelioma attorneys help asbestos-exposure victims pursue financial compensation.
- Asbestos was commonly used in various products and industries for its heat resistance and durability.
- Long Term exposure to asbestos can cause serious health complications such as Mesothelioma and Lung Cancer.
- If you have suffered health complications due to long term exposure to asbestos you may be entitled to compensation.
Asbestos is a dangerous toxin because its microscopic fibers are undetectable and enter the body to quietly wreak havoc on internal organs without your knowledge. Asbestos is odorless and tasteless, and the airborne particles are invisible. By the time symptoms do emerge, it is often too late—a terminal illness, such as mesothelioma, may have already taken hold and reached an advanced stage.
Workers exposed to asbestos during most of the past century knew they were working with asbestos, but the asbestos product manufacturers failed to inform the public that asbestos leads to disease, even though they knew for decades.
Some workers were exposed to asbestos without their knowledge, even after the public became aware of its dangers in the 1970s and 80s. Many of these workers only discovered they had been exposed to asbestos when they were diagnosed with mesothelioma and other illnesses.
If you’ve been diagnosed with mesothelioma, our knowledgeable Minnesota mesothelioma lawyers at Sieben Polk, P.A., can help you identify how you were exposed to asbestos and pursue financial compensation from the responsible companies.
How Are People Exposed to Asbestos?
People are exposed to asbestos through the inhalation or ingestion of invisible, airborne asbestos fibers that have been disturbed.
Asbestos is a group of silicate minerals that take on a fibrous, hair-like form that can be separated into individual strands and even woven into fabrics. It is a naturally occurring substance.
Asbestos is valued for its strength, flexibility, and lightness, but these are the same qualities that make it dangerous for humans. Due to their light weight, asbestos fibers quickly become airborne and spread throughout the environment when disturbed. Asbestos fibers can be disturbed by:
- Drilling or puncturing an asbestos-containing material
- Sanding or grinding asbestos-tainted products
- Sawing or breaking products that contain asbestos
- Wearing down products that contain asbestos through repeated use
- Weaving or mixing raw asbestos into another substance
- Mining asbestos or other minerals contaminated with asbestos
Airborne asbestos fibers are invisible, which means billions of them can fill a small space or spread over a large area, making it easy for humans to inhale them in large numbers. The body is unable to break down asbestos fibers due to their hard crystalline microstructure. As a result, they become embedded in the chest cavity and other parts of the body.
The most common type of asbestos is chrysotile, which generally occurs as long, thin, curly strands. This type of asbestos is the only type in the serpentine family of minerals. The other type of asbestos minerals are in the amphibole family. Asbestos in the amphibole family is thinner and more needle-like. It is considered more dangerous than chrysotile asbestos because it embeds more deeply in the tissues.
Health Risks of Asbestos Exposure
As it embeds inside the body, asbestos causes scarring, inflammation, and DNA damage over the course of decades, generally without any warning signs that irreversible damage is occurring. Ultimately, these processes lead to deadly diseases that generally do not show symptoms until they are very advanced.
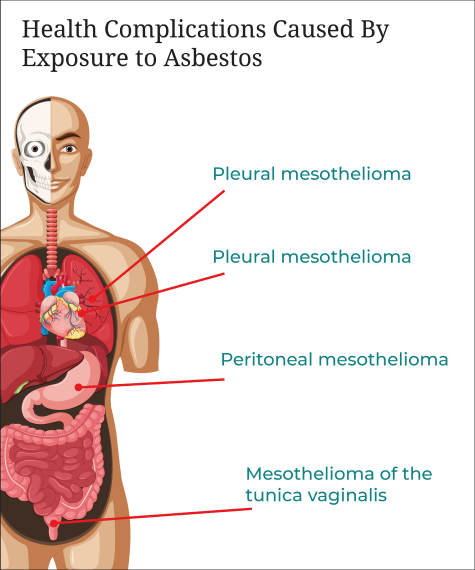
Health Complications Caused By Exposure to Asbestos
The most well-known condition caused by asbestos exposure is mesothelioma. Mesothelioma is the cancer of the mesothelium, a thin membrane that lines the cavities containing internal organs like the lungs and heart. It can occur as any of the following:
- Pleural mesothelioma – Cancer of the linings around the lungs and the most common form, accounting for 80 to 85 percent of cases.
- Peritoneal mesothelioma – Cancer of the membranes of the stomach, which accounts for 10 to 15 percent of cases.
- Pericardial mesothelioma – A rare form of mesothelioma that impacts the membrane that surrounds the heart.
- Mesothelioma of the tunica vaginalis – Also known as testicular mesothelioma, it affects the lining surrounding the testicles.
The average survival rate for mesothelioma is four to 18 months, but this varies according to the type of mesothelioma, its stage at diagnosis, and available treatment options. Some mesothelioma patients have lived ten years or more after diagnosis, but this is rare. According to the American Cancer Society, the five-year survival rate for malignant pleural mesothelioma is 12 percent.
Mesothelioma isn’t the only health complication that can stem from asbestos exposure. Exposure may also lead to the following health conditions:
- Lung cancer
- Asbestosis
- Cor pulmonale
- Constrictive pericarditis
- Pleural plaques
- Diffuse pleural thickening
- Pleural effusions
- Ovarian cancer
- Laryngeal cancer
- Pharyngeal cancer
- Stomach cancer
- Colorectal cancer
- Atelectasis
- Calcification of the pericardium


Where Does Asbestos Exposure Occur?
The most common source of asbestos exposure is the workplace, but it can occur almost anywhere. In 2021, Minnesota Governor Tim Walz declared September 26 Mesothelioma Awareness Day due to the prevalence of asbestos exposure in old homes, buildings, naval shipyards, construction sites, manufacturing plants, and beyond.
Occupational Asbestos Exposure
The most common and significant exposures to asbestos occur in the workplace. Workers who are exposed to significant levels of asbestos on a daily basis over an extended period have the highest risk of health complications. The occupations in Minnesota with the highest levels of asbestos exposure include:
- Foundry workers
- Construction workers
- Plumbers
- Electricians
- Oil refinery workers
- Taconite miners
- Vermiculite processors
- Paper mill workers
- Textile mill workers
- Steelworkers
- Auto mechanics
- Power plant workers
- Factory workers
- Firefighters
The Occupational Health and Safety Administration has also imposed strict regulations about how asbestos in the workplace should be handled and contained. The EPA also regulates the disposal of asbestos.
The OSHA allows workers to be exposed to a maximum of 0.1 fibers of asbestos per cubic centimeter over the course of an eight-hour workday. However, even this low level of asbestos poses hazards to human health.
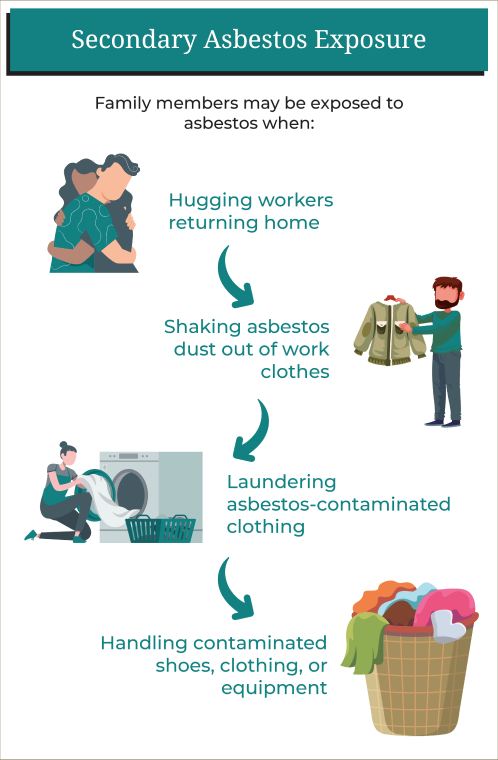
Secondary Asbestos Exposure
Secondary asbestos exposure can occur when family members are exposed to asbestos after workers carry home asbestos fibers on their clothing, shoes, skin, hair, tools, or equipment. Secondary asbestos exposure happens to be the most common source of mesothelioma in women.
Family members may be exposed to asbestos when:
- Hugging workers returning home
- Shaking asbestos dust out of work clothes
- Laundering asbestos-contaminated clothing
- Handling contaminated shoes, clothing, or equipment
Once asbestos enters the home, it can contaminate every room in the house. For example, it can fall off of clothing and land on furniture, carpets, and hard floors. Vacuuming or sweeping is likely to result in the fibers becoming airborne. Shaking work clothes can also release fibers into the air.
Laundering contaminated asbestos clothing can contaminate any other clothing washed with it, as washing machines cannot filter asbestos. This means the whole family could be wearing contaminated clothing on a daily basis.
Airborne asbestos can also enter heating, ventilation, and air conditioning systems. Microscopic asbestos fibers can easily penetrate most air filters. This allows asbestos to circulate throughout the home. Every time anyone sits on contaminated furniture, walks on a contaminated floor, or sweeps a contaminated area, the fibers may become airborne.
Asbestos in Products
Asbestos was commonly used in industrial products, building materials, and consumer goods from the 1930s until the late 1970s. While workers bore the primary brunt of asbestos exposure, no one was safe. Asbestos was found nearly everywhere, including in the following products:
- Building insulation
- Floor tiles in homes, schools, and public buildings
- Plumbing insulation
- Ductwork insulation
- Cement water pipes
- Roofing shingles and felt
- Siding
- Drywall and plaster
- Popcorn ceilings
- Textured paint
- Caulking
- Joint compound
- Textured paint
- Soundproofing materials
- Fire bricks
- Door insulation and gaskets in wood stoves
- Clothing irons
- Hair dryers
- Toasters
- Fire-resistant fabrics
- Soundproof curtains
- Theater curtains
- Pot holders and heating pads
- Protective clothing
- Decorative “snow” used around the holidays
- Filters in the Kent brand of cigarettes
Asbestos is generally only dangerous if it is not contained. However, even normal wear can damage asbestos. This is especially a concern in older buildings, where asbestos floor tiles are common. Other items prone to wear that could release asbestos include wood stove insulation, boiler insulation, and textiles.
Asbestos in adhesives, compounds, drywall, and insulation can become disturbed if it is drilled into, punctured, or otherwise damaged. This could happen even during minor activities like hanging pictures or installing cables. If you live in an older house, it is important to know its asbestos status before drilling into walls.
Some forms of asbestos are easily damaged even without damage or wear. This is known as friable asbestos. It is dangerous because it can become airborne if you touch it at all, or even when disturbed by a passing breeze. The most common sources of friable asbestos include popcorn ceilings, spray-on insulation, and fake snow.
Asbestos Exposure in the Military
If you have ever served in the United States military, your risk of mesothelioma is significantly higher than that of the general population. In fact, approximately 30 percent of all mesothelioma deaths in the United States happen to military veterans, even though they only represent eight percent of the population.
The risk is highest for Navy veterans. Asbestos was used widely in Navy and Coast Guard vessels as a result of being lightweight, resistant to fire, abundant, and inexpensive. Shipyard workers and sailors were at risk.
Asbestos was liberally used to line the keel of ships, as well as in bulkheads, boilers, engine rooms, pipe insulation, and electrical wiring. It was in such high demand for the Navy during World War II that the military prohibited most civilian use of asbestos in order to conserve as much as possible for the war effort.
Navy sailors weren’t the only personnel who faced asbestos exposure risks in the military. Members of all branches of the service did. Other sources of asbestos exposure in the military include the following:
- Brakes and friction parts on land vehicles and aircraft
- Gaskets in vehicle engines and machinery
- Building components in living quarters on military bases
- Buildings destroyed through fires and explosions during combat
Approximately 40,000 members of the Minnesota National Guard were deployed in the aftermath of the attack on the World Trade Center on September 11, 2001. Most served overseas, but many assisted in the cleanup of Ground Zero, where a toxic plume had taken over.
According to the International Journal of Disaster Risk Reduction, 5,000 tons of asbestos was found in the debris. The first death from mesothelioma stemming from the 9/11 terrorist attack occurred in March of 2006, less than five years after the attack. It is unusual for mesothelioma to develop so quickly. This may be a reflection of the extensive nature of the exposure at Ground Zero.
Environmental Asbestos
Environmental asbestos exposure occurs when asbestos is present in the surroundings even though you are not handling an asbestos product. This may stem from naturally occurring asbestos, such as in areas surrounding the taconite mines in northeastern Minnesota that experience erosion.
Environmental asbestos exposure can also be the result of human activity. According to findings by the Minnesota Department of Health, urban Minnesotans who live downwind from factories that produce asbestos products have contracted mesothelioma even though they are not exposed to asbestos in their occupations.
Residents of Duluth and other cities that source their water from Lake Superior are exposed to asbestos in the water supply. In 1973, the EPA found high levels of asbestos in Lake Superior. It is there because Reserve Mining, a taconite mining company, disposed of asbestos-tainted waste products in the lake.
The court ordered the plant to shut down in 1974, but this threatened to devastate the area economically. Reserve Mining appealed, and a higher court issued a stay, allowing the company to continue disposing of taconite waste products in Lake Superior until an alternative land-based disposal system could be developed. This didn’t happen until 1980. Reserve Mining went bankrupt in 1986.
Asbestos Exposure in Minnesota
Asbestos exposure has taken a significant toll on the North Star State. In 2020 alone, 66 Minnesotans were diagnosed with mesothelioma, and 63 died. From 1999 to 2020, Minnesota was only one of seven states in which the age-adjusted death rate for mesothelioma in women exceeded six per million.
Minnesota Congressman Bruce F. Vento died of mesothelioma in 2000 after serving for 23 years. He was a champion of environmental causes. He blamed his asbestos exposure on his early career in a brewery and a refrigerator plant.
The impact of asbestos exposure continues to affect Minnesotans to this day, both as a result of exposure that occurred decades ago and ongoing asbestos exposure.

The Construction Industry
Workers in the construction industry at high risk of asbestos exposure include drywallers, carpenters, demolition workers, roofers, and floor tile workers. During the peak of asbestos use, these workers handled asbestos daily while building residential and commercial structures until the late 1970s. Construction workers were exposed to asbestos through the following products and more:
- Cement
- Adhesives
- Joint compound
- Flooring adhesive
- Floor tiles
- Roof shingles and felt
- Drywall
- Paint
- Wall and ceiling plaster
- Building insulation
- Pipe and duct insulation
- Electrical wiring insulation
- Spray-on insulation
- Popcorn ceilings
- Ceiling tiles
Although asbestos is no longer used today, construction workers can still be exposed during building renovations and demolitions. This is why the Minnesota Department of Health requires contractors to undergo asbestos inspections before initiating building demolitions or renovations. However, these requirements primarily apply to commercial buildings.
Construction workers may be exposed to asbestos if contractors fail to comply with state inspection and abatement requirements and during renovations or demolitions of residential properties built before 1980.
Taconite Iron Ore Mining in Rural Minnesota
The University of Minnesota has found an alarming trend in northeast Minnesota, in which the risk of mesothelioma in men in the seven-county Arrowhead region from 2000 to 2009 was double the rate of men outside this region. In particular:
- Men in Carlton County had the third highest mesothelioma mortality in the nation at 55.1 deaths for every one million people.
- St. Louis County had the 21st highest rate at 25.1 mesothelioma deaths per million.
- The national average is 11.1 mesothelioma deaths per million.
The seven counties are located in the Mesabi Iron Range, where taconite mining is a major industry. These mines are known to contain non-asbestiform elongate mineral particles (EMPs), which are composed of the same minerals as asbestos but lack the same strength and durability.
Whether these EMPs are responsible for the increased risk of mesothelioma in taconite workers has been a source of controversy among scientists. Studies in other regions with similar EMPs did not find an increase in mesothelioma.
According to a final report issued in 2014, taconite miners had triple the risk of developing mesothelioma, and workers with above-average levels of exposure to these EMPs had nearly twice the risk of mesothelioma compared to those with below-average exposure. Workers with above-average exposures also suffered a higher incidence of lung scarring, which can lead to asbestosis.
In 2019, scientists concluded that asbestos in the insulation used in the mines may be the true cause of the heightened mesothelioma incidence among taconite miners. The increased risks are significant. According to the report:
- Approximately 1.4 of every 1,000 adults who live to age 80 contract mesothelioma.
- Approximately 3.3 of every 1,000 taconite miners with 30 years of exposure who live to age 80 contract mesothelioma.
The mining jobs with the highest levels of exposure, according to the University of Minnesota study, are pellet plant welders, fine crusher oilers, coarse crusher laborers, and coarse crusher maintenance mechanics. However, many other miners also suffer from mesothelioma.
Exposure to EMPs or asbestos may occur during the intense processes that occur when iron ore is extracted from taconite. Explosives are used to blast it into small pieces. It is then crushed into smaller pieces after being transported to a processing plant. The iron is separated using powerful magnets, then dried, heated, and cooled, resulting in pellets. All of these processes can release asbestos and EMPs.
Asbestos Exposure at Factories and Plants
Taconite mining isn’t the only occupation in Minnesota’s Arrowhead region that contributed to the high rate of mesothelioma. Carlton County, which had the third highest mesothelioma death rate in the country, was home to Conwed Corporation, a manufacturer that produced asbestos ceiling tiles and other products.
A 1988 study by the Minnesota Department of Health found that 28 percent of the 1,100 former Conwed workers studied had developed lung abnormalities consistent with asbestos exposure. Most workers were exposed from 1958 to 1974.
Unfortunately, Conwed is not unique. Asbestos exposure at Minnesota job sites in the manufacturing sector was common during this period. Workers at the following companies were also exposed:

The W.R. Grace Western Mineral Plant in Minneapolis processed vermiculite from a contaminated mine in Libby, Montana. At the time, the Libby mine was the largest vermiculite supplier in the world. It was contaminated with tremolite asbestos, a member of the amphibole family.
During processing, vermiculite was heated until it popped like popcorn, releasing a light, porous substance. The news about the contamination only reached the public in 1989. Libby vermiculite was marketed under the trade name Zonolite to be used for home insulation. Workers at the Minneapolis plant could be exposed during processing or while installing or removing the insulation.
Local residents were allowed to take piles of vermiculite waste materials home for free to use in their gardens, driveways, and other projects. Children sometimes played in piles of it. This is where the highest concentrations of asbestos were found. Minnesotans are still developing mesothelioma and other asbestos-related illnesses due to asbestos in Libby vermiculite.
Power Plants
Workers may be exposed to significant levels of asbestos in Minnesota power plants. Among the key qualities of asbestos that made it attractive for industrial uses were its fire resistance, resistance to corrosion, and non-conductive nature. Asbestos was used in the walls, cement, machine gaskets, pipe insulation, boilers, and other areas of power plants to insulate against fire, heat, and electricity.
Workers at the following power plants have experienced asbestos exposure:
Getting Financial Help For Your Asbestos-Related Disease
If you’ve been diagnosed with mesothelioma or any other asbestos-related illness, you may be able to recover substantial compensation. The companies that mined products contaminated with asbestos or manufactured products that contained asbestos can be held liable for your financial losses, pain and suffering, and mental anguish.
Our experienced mesothelioma lawyers in Minnesota can help you pursue damages through one or more of the following avenues:
Most of the companies responsible for your asbestos exposure knew their products could cause deadly diseases, but they intentionally withheld this information from the public and even colluded to keep it secret for as long as possible to protect their profits.
Even today, these companies refuse to do the right thing. Instead, they fight claims by the people they harmed. They are large, wealthy corporations, and it takes an experienced mesothelioma lawyer with significant resources to hold them accountable. Don’t try to stand up to these powerful companies on your own. Call us today at (651) 437-3148 to schedule a free consultation.
Asbestos Exposure FAQs
Below are answers to frequently asked questions about asbestos exposure.
Shockingly, asbestos has not been completely banned in the United States. The EPA attempted to ban all uses of asbestos in 1989. However, the asbestos industry’s commitment to profit at the expense of worker safety caused resistance as the industry fought the ban in court. In 1991, the ban was largely overturned in federal court. Only the following products remained banned:
- New products containing asbestos
- Asbestos flooring felt
- Asbestos rollboard
- Specialty paper made with asbestos
- Corrugated asbestos paper
The only other asbestos products banned by the EPA are spray-applied asbestos, pipe insulation, boiler insulation, and hot water tank insulation.
However, the EPA has made progress. It issued a final rule in 2019 that prohibits the reentry of discontinued asbestos products back into commerce unless they first undergo an EPA evaluation. Such an evaluation is unlikely to authorize asbestos products, and even if it does, the sale or use of asbestos would likely be extremely limited.
In April of 2022, the EPA again issued a Proposed Ban of Ongoing Uses of Asbestos, which would essentially ban its use in the United States. This has yet to be approved.
If you suspect you have asbestos in your home, don’t attempt to remove it. If it is asbestos, moving it will release fibers into the air. The only way to confirm or rule out that the suspected area contains asbestos is by having it tested by a state-certified asbestos inspector.
If the inspector confirms you have asbestos, you should seek advice from a professional asbestos abatement company. If the asbestos is encased in an undamaged material, it may be safer to leave it in place. However, the asbestos may need to be removed in the following circumstances:
- It is a friable form of asbestos that may already be releasing airborne fibers.
- The material that encases the asbestos has been damaged.
- You intend to renovate the part of your house where the asbestos is located.
In some cases, it may be safer and less expensive to repair the material that encases the asbestos. Asbestos abatement is a laborious process that should only be performed by a qualified asbestos abatement company. You should never try to remove asbestos yourself because it could contaminate your home.
There is no safe level of asbestos. You can develop mesothelioma or other asbestos-related diseases as a result of a one-time exposure to a low level of asbestos. However, the risk is low in this instance. The majority of people who contract asbestos diseases are workers with long-time heavy exposures.
Don’t panic. Almost everyone has been exposed to asbestos, but not everyone develops an illness. If you know or suspect you have been exposed, you should mention it to your doctor so that this information becomes part of your medical record. This could result in an earlier diagnosis if you develop an asbestos disease.
There are no uniform screening tests that can predict whether you will develop mesothelioma or other diseases, but you may benefit from having annual chest X-rays and lung function tests. Mesothelioma and lung cancers both have a longer life expectancy and more treatment options with earlier detection.
If you smoke, you should quit. Multiple studies have consistently shown that when asbestos exposure is combined with smoking, the risk of lung cancer increases by 50-fold.
Being exposed to asbestos doesn’t guarantee that you will get mesothelioma or another asbestos-related condition. It is merely a risk factor. Before you can file any type of civil action, you must have proof that you suffered harm. An increased risk of developing an illness alone is not considered harm in civil claims in Minnesota.
You must have proof of a diagnosis before you can file a lawsuit, trust fund claim, or VA claim. If you do have a diagnosis, you should reach out to one of our skilled mesothelioma lawyers as soon as possible because state law limits the time you have to file your claim.


Helping Minnesotans Exposed to Asbestos for Over 50 Years
Our award-winning Minnesota mesothelioma attorneys have over 200 years of combined experience helping asbestos exposure victims throughout the Upper Midwest. No one understands the legal landscape of Minnesota like we do.
Our team’s unwavering dedication to our clients is reflected in the results, testimonials, and our track record of success. We have won more than $840 million in compensation for Minnesotans in asbestos cases. When you hire our firm, you can expect personalized legal services and the resources you need to protect your rights.
Because we operate on a contingency fee basis, you pay nothing unless we secure compensation on your behalf. Contact us today to schedule your free consultation with an experienced Minnesota asbestos lawyer.
Sources:
Related Reading:
Sources:
Www.health.state.mn.us, www.health.state.mn.us/communities/environment/asbestos/homeowner/asbinhomes.html.
Regulatory Toxicology and Pharmacology, vol. 52, no. 1, Supplement, 1 Oct. 2008, pp. S31–S39, www.sciencedirect.com/science/article/abs/pii/S0273230007001390, https://doi.org/10.1016/j.yrtph.2007.09.019.
Washington Post, 11 Oct. 2000, www.washingtonpost.com/archive/local/2000/10/11/minnesota-rep-bruce-vento-dies/aa60a82d-f222-479a-bc0a-247cddc8fd08/.
Osha.gov, 2014, www.osha.gov/laws-regs/standardinterpretations/2014-11-05-1. Accessed 11 Jan. 2024.
Gis.cdc.gov, June 2021, gis.cdc.gov/Cancer/USCS/#/AtAGlance/.
Who Developed Mesothelioma Minnesota Department of Health Chronic Disease and Environmental Epidemiology. 2003.
Www.health.state.mn.us, www.health.state.mn.us/communities/occhealth/data/mesothelioma.html.
S E P T E M BE R 1 9 9 3 ; U P DA T E D D E c E M BE R 2 0 2 1.
International Journal of Disaster Risk Reduction, vol. 48, Sept. 2020, p. 101563, https://doi.org/10.1016/j.ijdrr.2020.101563. Accessed 15 May 2022.
MMWR. Morbidity and Mortality Weekly Report, vol. 71, no. 19, 13 May 2022, pp. 645–649, https://doi.org/10.15585/mmwr.mm7119a1. Accessed 15 Sept. 2022.
Mn.gov, mn.gov/governor/assets/09.26.21%20Mesothelioma%20Awareness%20Day%20Signed_tcm1055-500597.pdf. Accessed 11 Jan. 2024.
Cancer Management and Research, vol. Volume 11, May 2019, pp. 4997–5012, https://doi.org/10.2147/cmar.s205723.
European Respiratory Journal, vol. 50, no. suppl 61, 1 Sept. 2017, erj.ersjournals.com/content/50/suppl_61/PA4294, https://doi.org/10.1183/1393003.congress-2017.PA4294.
School of Public Health, 25 Jan. 2019, www.sph.umn.edu/news/mesothelioma-in-taconite-workers-most-likely-due-to-asbestos/. Accessed 11 Jan. 2024.
Penn Medicine – Abramson Cancer Center, www.pennmedicine.org/cancer/types-of-cancer/mesothelioma/prognosis.
Www.cbsnews.com, 1 Dec. 2014, www.cbsnews.com/minnesota/news/researchers-present-final-report-on-taconite-risks/. Accessed 11 Jan. 2024.
A Review of Epidemiology Literature and Study Recommendations on Asbestos Performed under Contract from the Minnesota Department of Health for the Minnesota Regional Copper-Nickel Study Environmental Impact Task Force. 1977.
Lung Cancer International, vol. 2017, 2017, www.ncbi.nlm.nih.gov/pmc/articles/PMC5292397/, https://doi.org/10.1155/2017/2782590.
Www.cancer.org, www.cancer.org/cancer/types/malignant-mesothelioma/detection-diagnosis-staging/survival-statistics.html.
EXECUTIVE SUMMARY 2 ASBESTOS EXPOSURE MOST COMMON in NAVY.
– Minnesota National Guard. ngmnpublic.azurewebsites.us/twenty-years-later-minnesota-remembers-9-11/. Accessed 11 Jan. 2024.
Www.epa.gov, 12 Mar. 2013, www.epa.gov/asbestos/asbestos-ban-and-phase-out-federal-register-notices.
US EPA, 12 Mar. 2013, www.epa.gov/asbestos/epa-actions-protect-public-exposure-asbestos.
Www.health.state.mn.us, www.health.state.mn.us/communities/environment/asbestos/workprac/index.html. Accessed 11 Jan. 2024.
